Pran
Your health, reimagined.
Pran simplifies wellness through an innovative device and smart cartridge system—bringing convenience and care into your daily routine, painlessly.
Pran
Your health, reimagined.
Pran simplifies wellness through an innovative device and smart cartridge system—bringing convenience and care into your daily routine, painlessly.

Cartridge
Cartridge
The cartridge holds the liquid formulation (e.g., NAD+ or other treatments). It's designed for easy replacement, leak prevention, and optimal flow to the electrospray nozzle, ensuring smooth and efficient atomization delivery.

Cartridge explode diagram
The exploded diagram highlights three essential components: the mouthpiece for aerosol intake, the cartridge reservoir for holding the therapeutic liquid, and the electrospray system that creates fine droplets through ionization for optimal delivery.

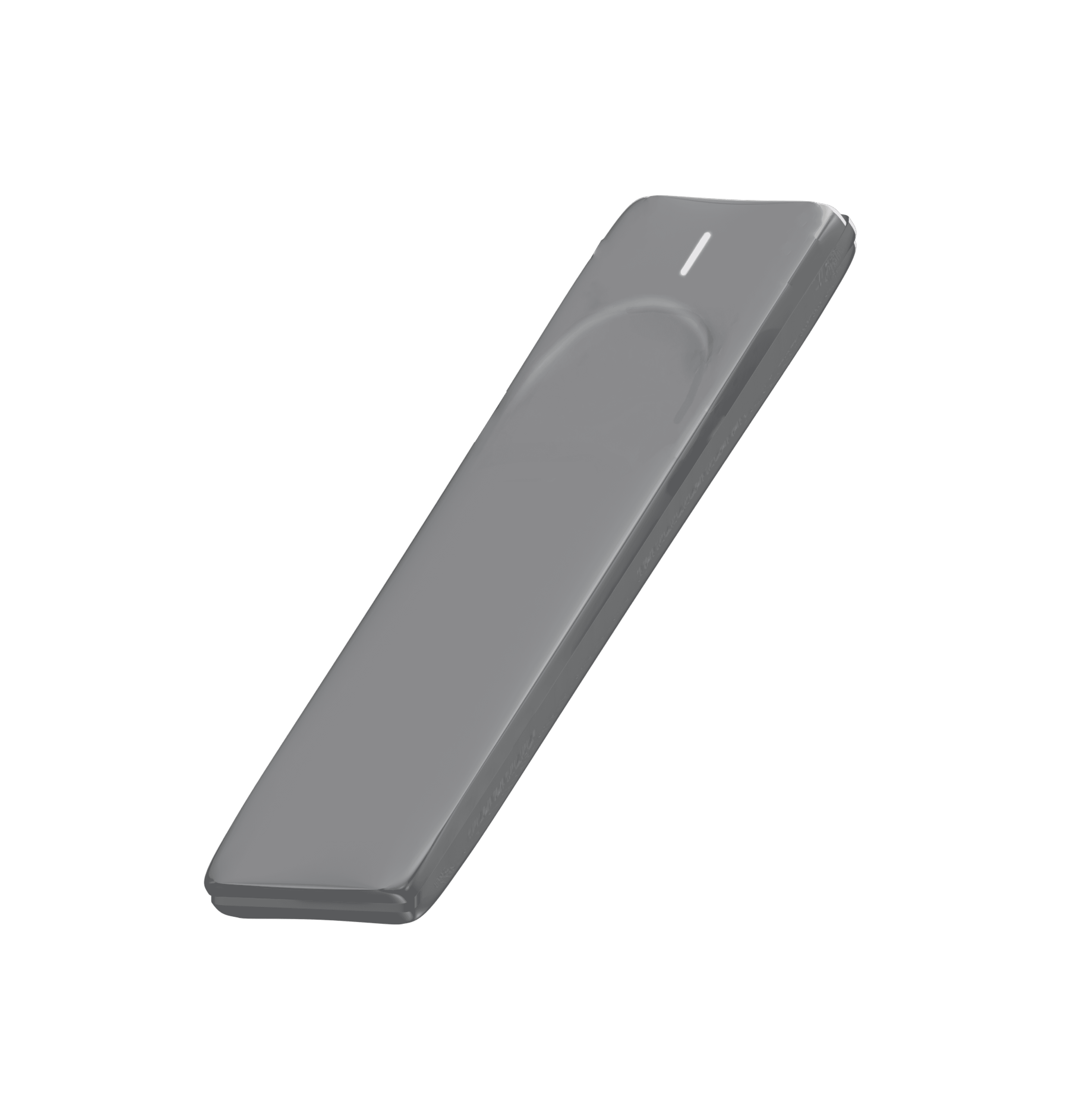
Device
Implementation Plan
2025
R&D & Patent Phase
Discover engineering process, file PCT patent, define target markets, and file national patents.
2025-2026
ISO & Regulatory
Research at ISO 17025 lab, pass ISO 10993 tests, and setup ISO 13485 factory for manufacturing standards.
2026-2027
Preclinical Studies
Conduct preclinical trials to establish safety and efficacy data before human testing.
2027-2029
Phase I Clinical Trial
First human testing phase to evaluate safety, dosage, and side effects in small groups.



